The Ding Lab is deeply connected to many large-scale cancer research consortium projects.
Click to learn more about each project!
Human Tumor Atlas Network (HTAN)
The Human Tumor Atlas Network (HTAN) project at Washington University integrates cutting-edge technologies to study breast and pancreatic cancers. It is a large-scale effort to understand the life histories of tumors, including how normal cells become cancerous; how the cancer evolves in response to treatment; and what changes must occur for the tumor to become resistant to therapy and spread. The project takes advantage of the most advanced technologies, such as single cell sequencing, making possible studies that could not be conducted until very recently. The Ding Lab plays a leading role in this effort at Wash U, performing a variety of bioinformatics and imaging analyses.
PE-CGs
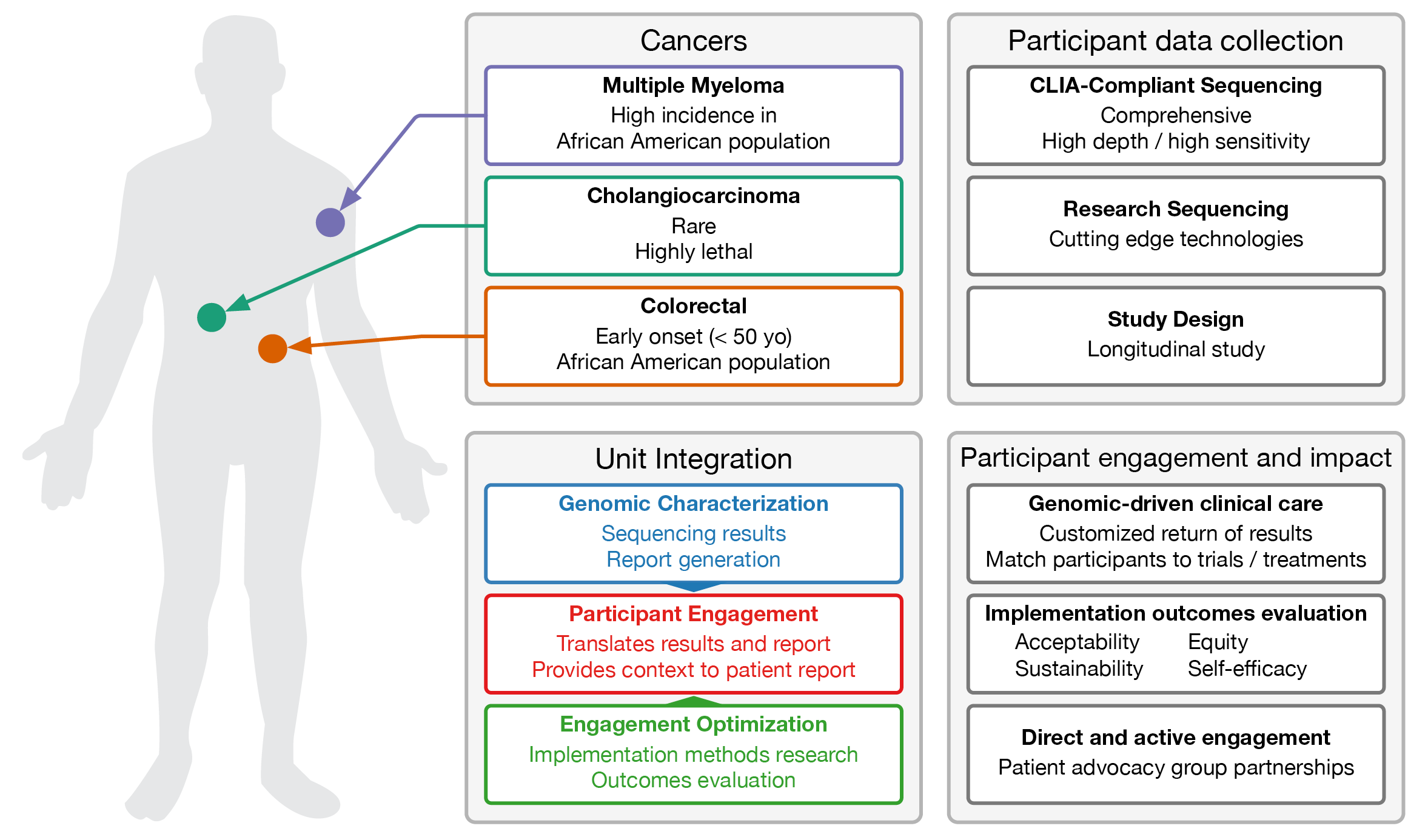
Washington University is part of the The National Cancer Institute's (NCI) Participant Engagement and Cancer Genome Sequencing (PE-CGS) Network, itself part of the NCI Cancer Moonshot Initiative. The program is developing strategies and processes to engage cancer patients as participants in research and in their own treatment, especially among cohorts under-represented in research and under-served minority patients. The Washington University component focuses on African American patients having colorectal cancer and multiple myeloma, as well as all patients having cholangiocarcinoma, a rare cancer of the bile ducts. Patients will have both their tumor and unaffected normal genomes sequenced and compared to shed light on cause and possible treatment options. In consultation with medical geneticists, these sequencing results will actually be part of return-of-results (RoR), marking another conspicuous step toward personalized medicine.
Sennet
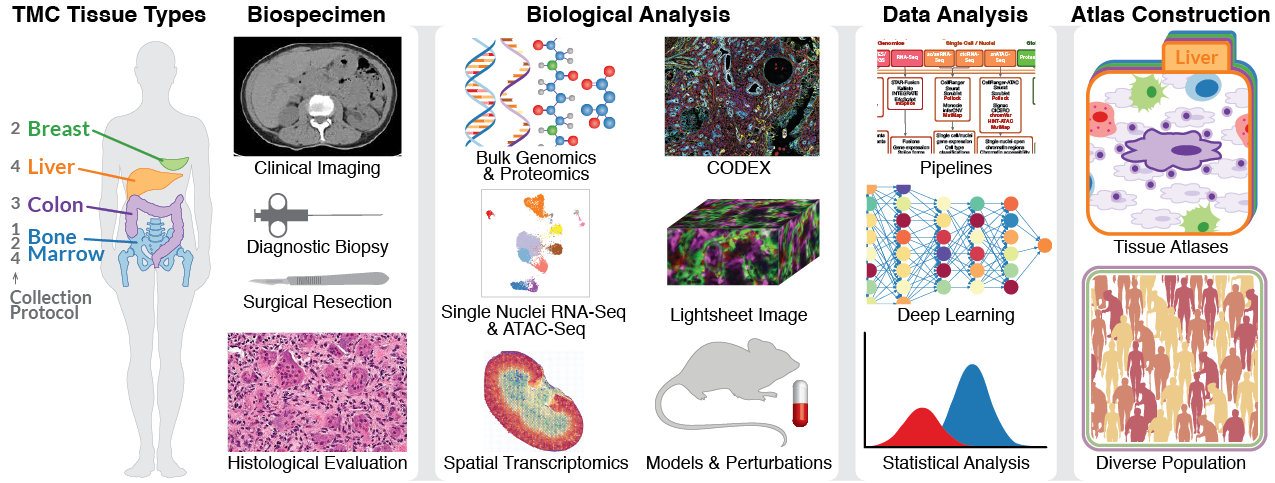
The Ding Lab is part of the National Institutes of Health (NIH) Cellular Senescence Network (SenNet), a large effort involving more than a dozen research centers aimed at advancing understanding of "old" cells that have lost certain functionality, like the ability to further divide. Senescence has far-ranging biomedical implications for aging, tissue repair, and diseases like cancer. Our efforts within the SenNet network focuses on four tissue types, namely breast, liver, colon, and bone marrow, generating and analyzing comprehensive molecular and imaging data. The project aims to build multi-dimensional, high resolution atlases of senescent cells and senescence associated secretory phenotype (SASP), and signatures of senescence in different tissues and ages. Markers and signatures will be cross validated in mouse models and in additional human tissues.
The Cancer Genome Atlas (TCGA)
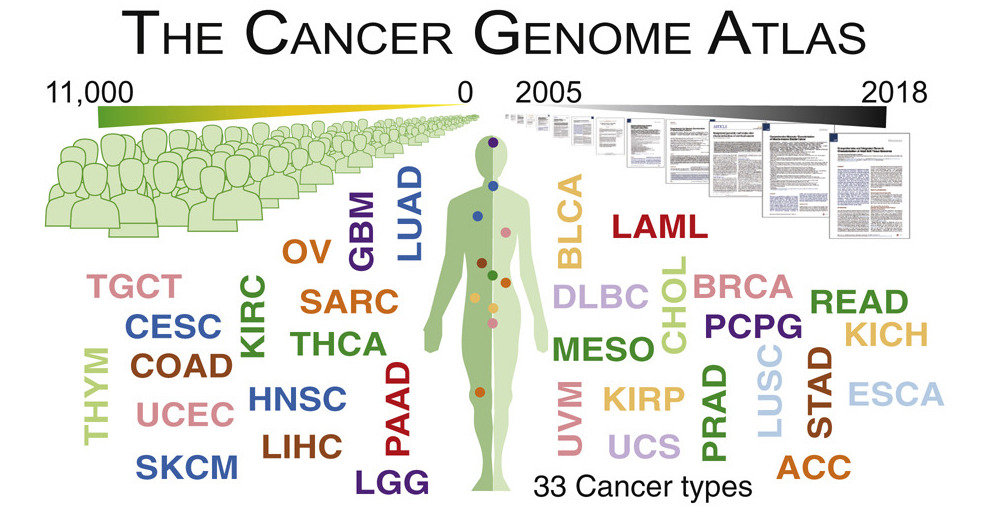
The Cancer Genome Atlas (TCGA) is a foundational large-scale cancer genomics data set which has revolutionized the way cancer is studied, leading to new understandings of cancer biology and treatment options. Since 2006, data from over 11,000 patients representing 33 cancer types, including tumor and normal whole exome and whole genome sequencing, RNA and protein expression, and clinical characteristics, has come to represent the first phase of cancer genomics. The Ding Lab served as a leader of several TCGA projects, including methods for identifying cancer driver genes, detecting germline variants, and integrating genomics and proteomics data, all at a large computational scale. The lessons learned and experience gained as TCGA leaders have given the Ding Lab a unique role in the next phase of cancer genomics and proteomics.
GDAN
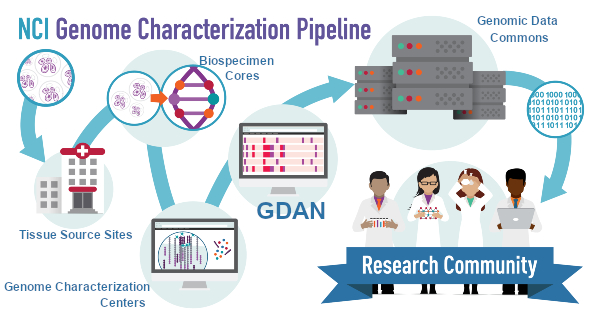
The Genomic Data Analysis Network (GDAN) follows on the heels of the NCI Center for Cancer Genomics’ TCGA program. It brings together a large collection of research teams, each having specialized areas of investigative expertise, to continue large-scale processing and analysis of tumor genomic data across many cancers. The Ding Lab has been a GDAN member group since 2016, playing an active role in several of the GDAN Analysis Working Groups (AWGs), namely the ALCHEMIST lung cancer trials, the Clinical Trial Sequencing Project for DLBCL (a lymphoma), TGCT testicular cancer, and the ATAC-seq working group. We continue to take on new responsibilities within the GDAN, including for the new AWGs for the Multicentric Italian Lung Detection (MILD) project, KIRC kidney cancer project, and the Human Cancer Model Initiative (HCMI) program for development of new cancer models.
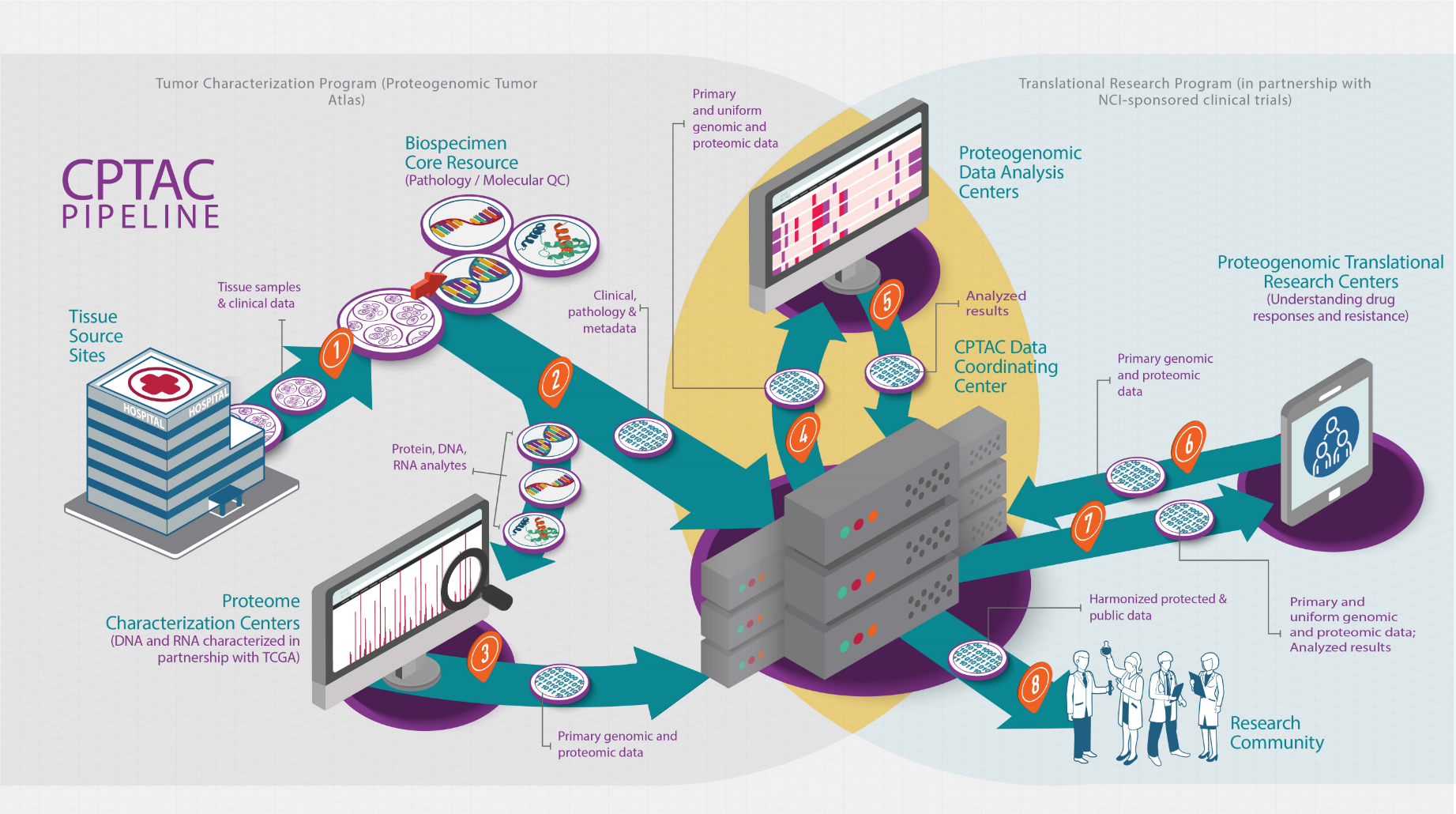
Clinical Proteomic Tumor Analysis Consortium (CPTAC)
Image source: https://proteomics.cancer.gov/programs/cptac
The NCI Clinical Proteomic Tumor Analysis Consortium (CPTAC) is the first large-scale project to integrate genomics and proteomics data, with the goal of understanding of cancer driver events and developing novel therapies. CPTAC was launched in 2011 with pioneering proteogenomic studies of breast, ovarian and colorectal cancers (CPTAC2) and now has expanded to characterize ten additional cancer types (CPTAC3). CPTAC3 utilizes state-of-the-art shotgun protein identification and quantification by mass spectrometry to collect protein, phosphoprotein, acetylated protein, and glycoprotein data. The integration of several omics data – genomics, transcriptomics, proteomics, phosphoproteomics etc. – provides power to address issues of cancer therapeutic resistance and toxicity by elucidating cancer-relevant pathways through post-translational modifications. As one of CPTAC3’s Proteogenomic Data Analysis Centers, the Ding lab contributes to genomic data processing and analysis and, at the same time, is actively building expertise in proteomics and phosphoproteomics.
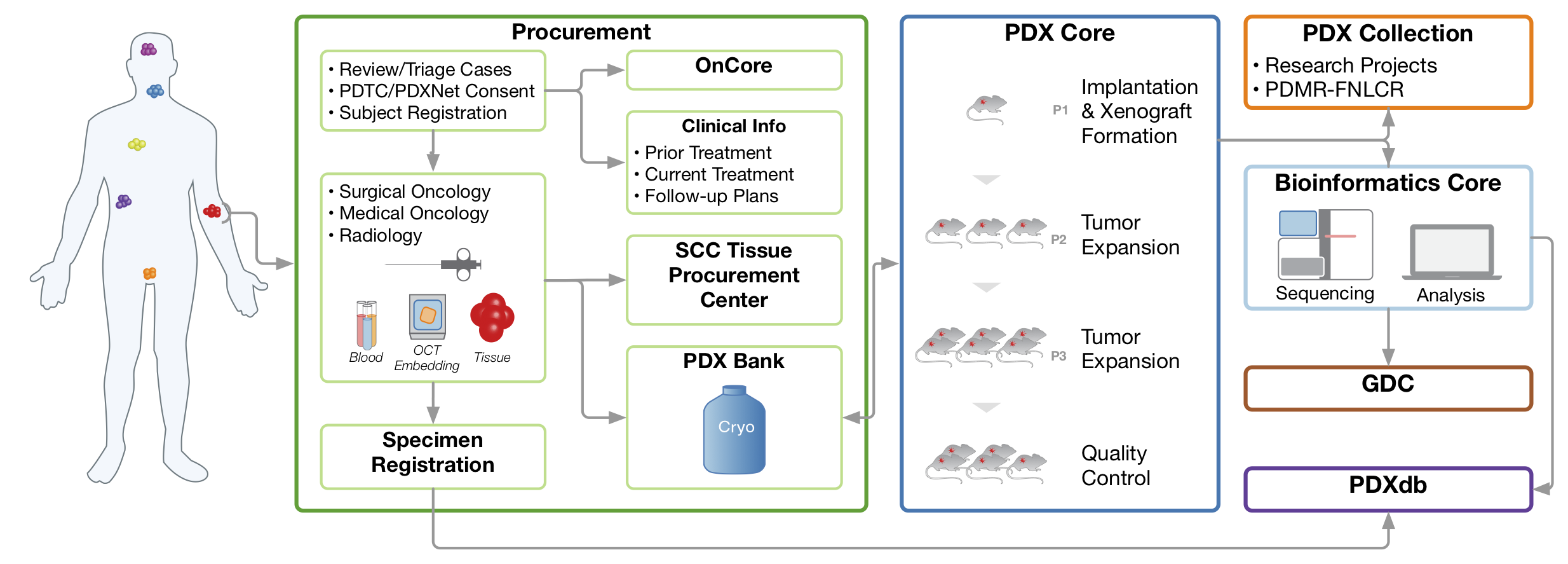
PDX Development and Trial Centers Research Network (PDXNet)
The National Cancer Institute launched the PDX (patient-derived xenografts) Development and Trial Centers Research Network (PDXNet) in September 2017 to accelerate translational research using PDX datasets. The overall goal of work at Wash U is to exploit the translational potential of PDX models for evaluating the response of various treatments in models with specific molecular characteristics. Work in the Ding Lab includes development of bioinformatics algorithms for the genomic and proteomic characterization and analysis of PDX models and the development of databases to support this initiative.
Multiple Myeloma (MM)
We are deeply involved in collaborations at WashU aiming to better understand multiple myeloma, both the mechanisms of disease progression and the advancement of treatment options. By integrating the resources and expertise of clinical research teams at the Washington University School of Medicine and the Multiple Myeloma Research Foundation (MMRF), our investigations promote the discovery druggable targets by using cutting-edge genomics and proteomics technologies. In addition, major efforts from our lab contribute to understanding the dynamic relationship between the tumor and microenvironment in multiple myeloma.
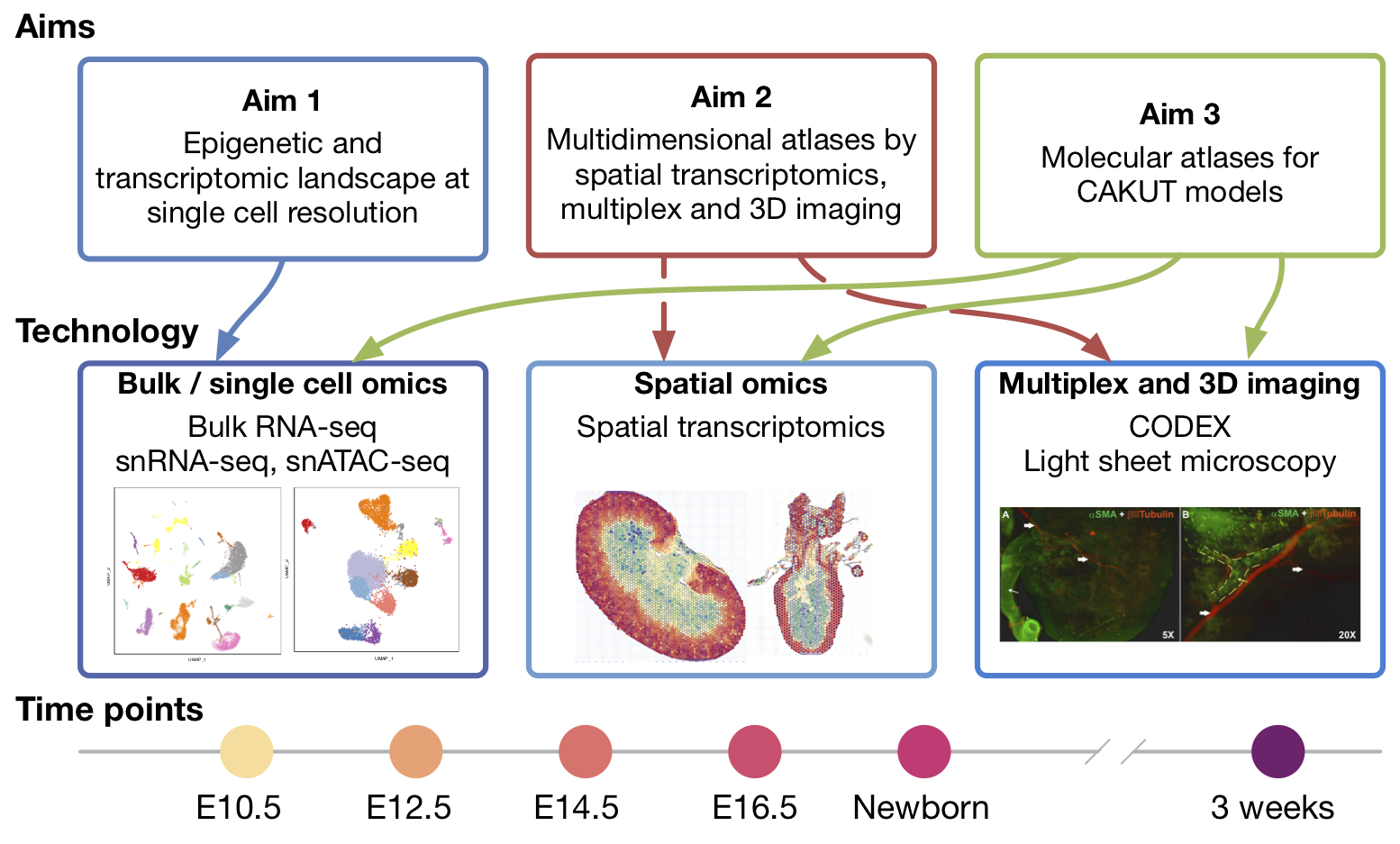
GUDMAP
One of the newest large-scale projects Washington University researchers have joined is the Genito-Urinary Development Molecular Anatomy Project (GUDMAP), which is administered by the National Institute of Diabetes, Digestive, and Kidney Diseases (NIDDK). Generally speaking, this project focuses on biomedical implications of molecular signaling cascades that affect development and the biomedical implications of signaling errors, including congenital anomalies of kidney and urinary tract (CAKUT). Our particular effort targets structures and organs that have not yet been investigated extensively and it seeks to build spatially detailed molecular atlases. We are applying single cell omics, spatial transcriptomics, multiplex imaging, lightsheet microscopy, and computational high resolution multi-omics. A major component is atlas-building for CAKUT mouse models at several key developmental points spanning gestation up to 3 weeks of age.
OTher Ding Lab PARTNERSHIPS
Pediatric Cancer Genome Project (PCGP): A collaboration between St. Jude Children's Research Hospital and Washington University, aimed at understanding the genetic origins of childhood cancers
http://explore.pediatriccancergenomeproject.org/
International Cancer Genome Consortium (ICGC): A project that obtains a comprehensive description of genomic, transcriptomic and epigenomic changes in 50 different tumor types and/or subtypes which are of clinical and societal importance across the globe
1000 Genomes Structural Variation Project: A collaboration to characterize and detect genomic structural variants existing in the human genome